Products
Lumbar and Cervical Inter-body Fusion Devices made with PEEK-OPTIMA®
Click on a thumbnail above to zoom image
-
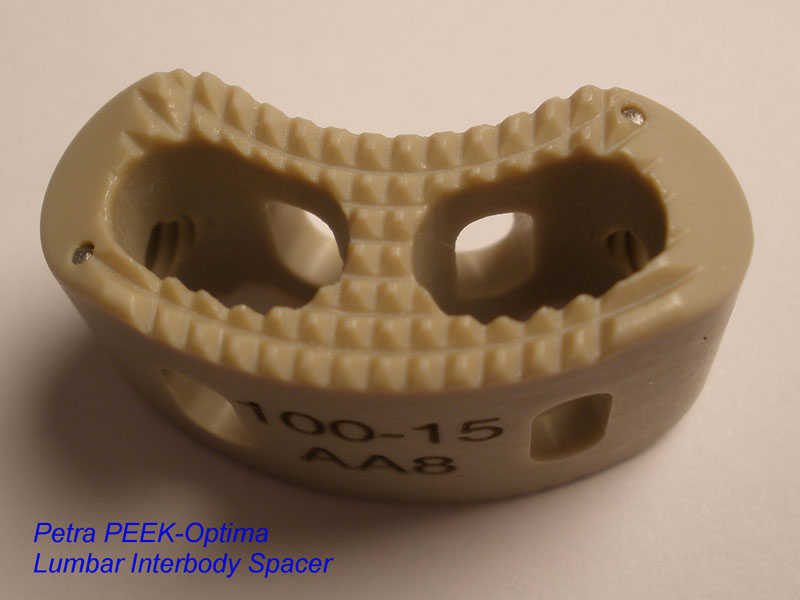
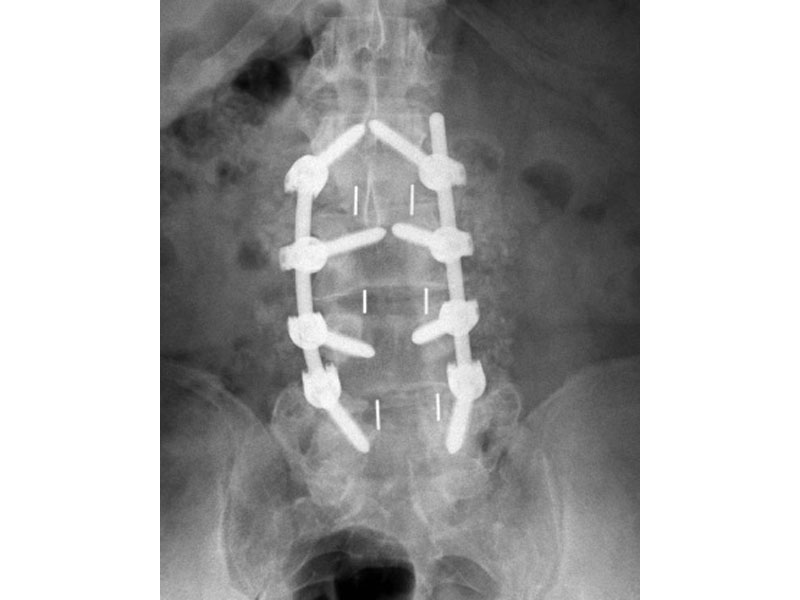
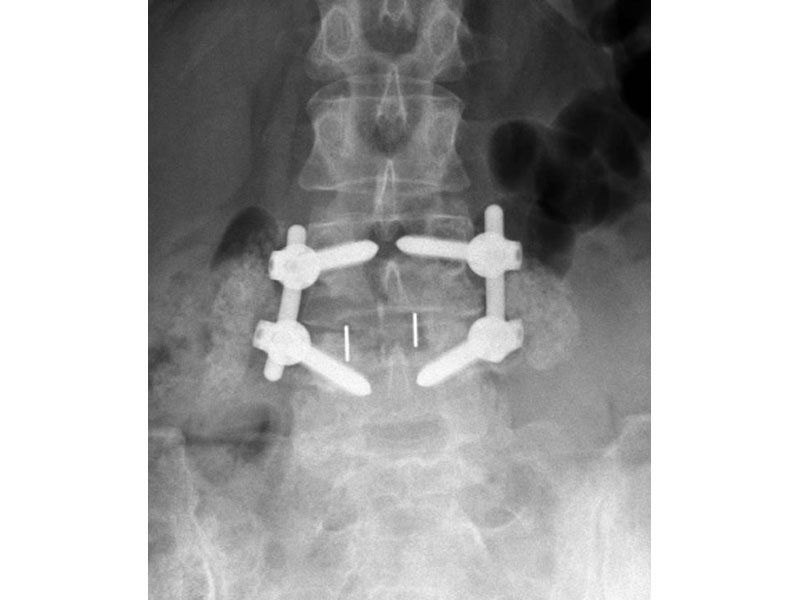
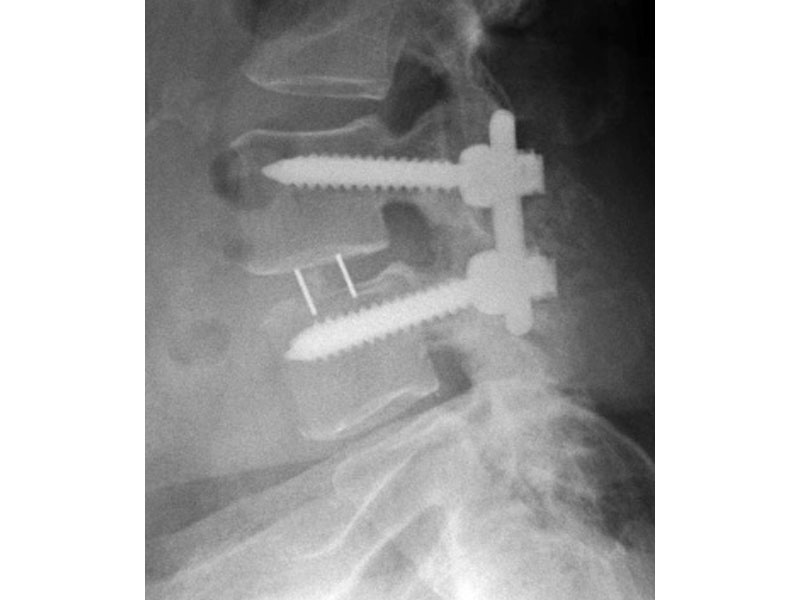
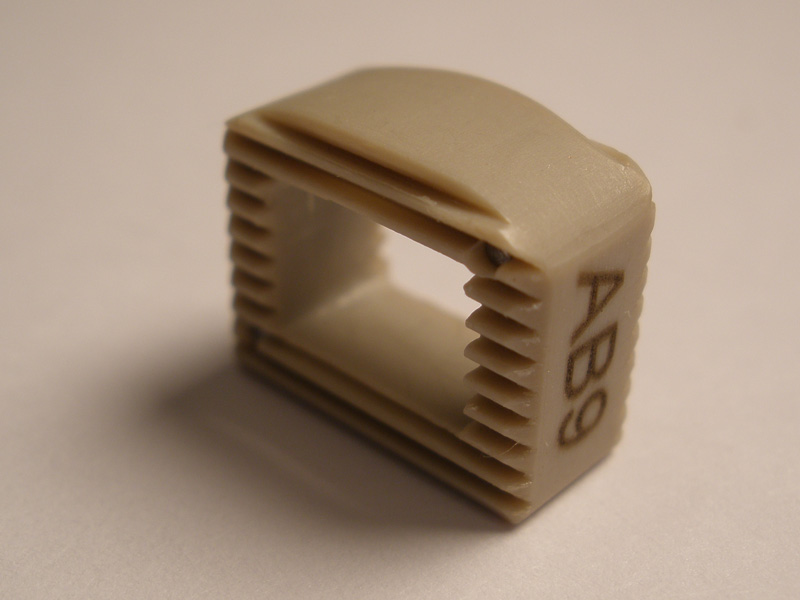
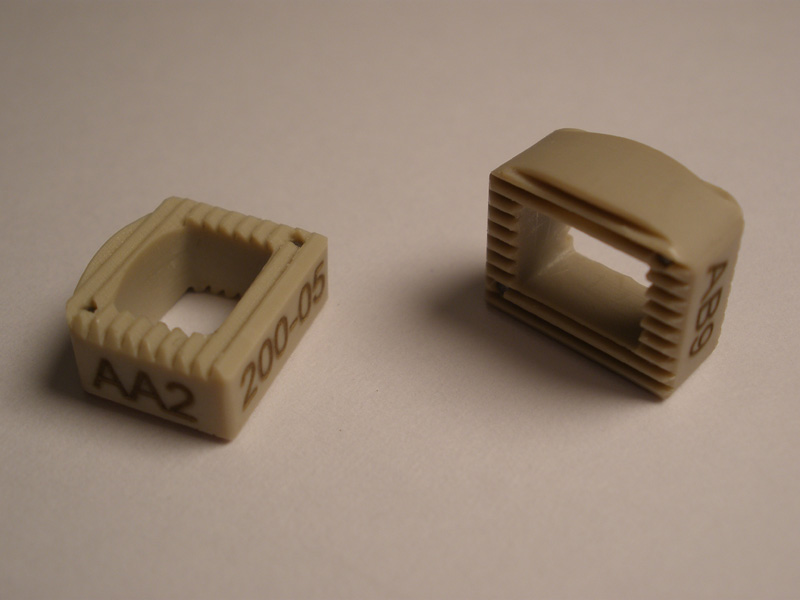
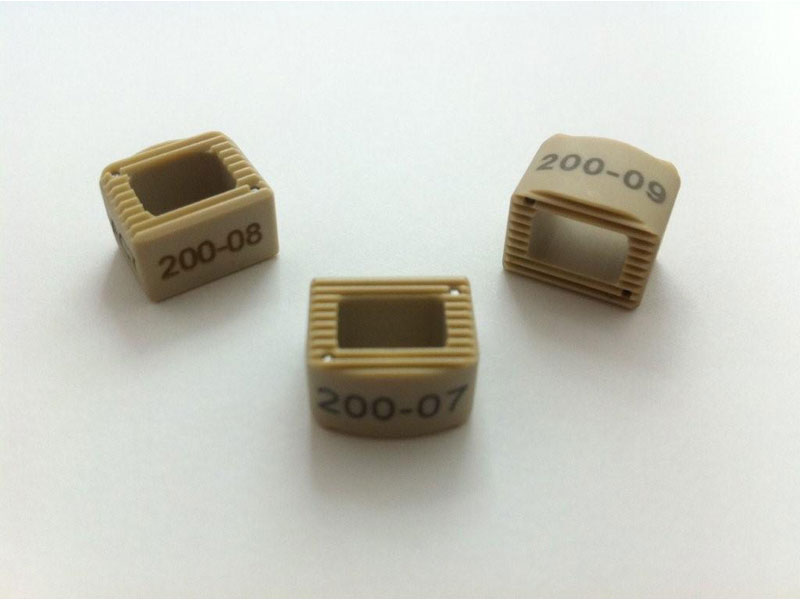
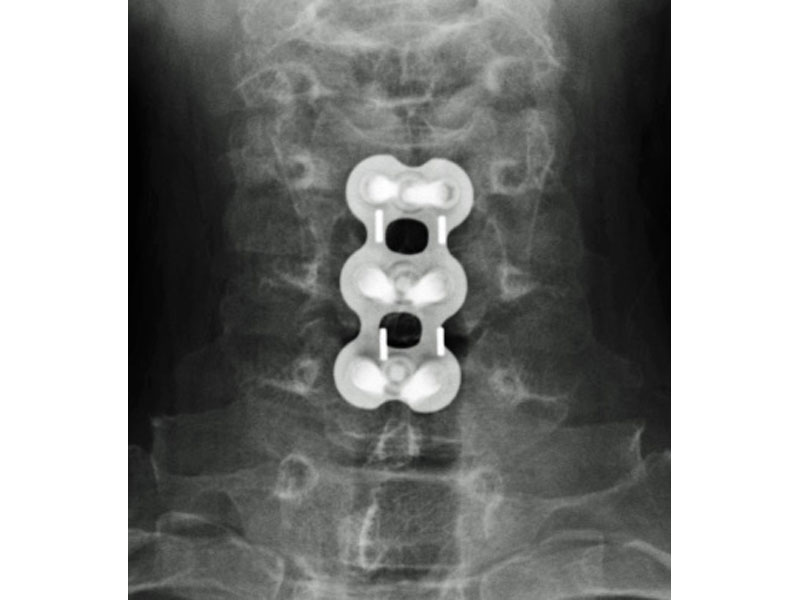
Calvary Lumbar Interbody Fusion Devices
- PEEK-OPTIMA polymer with proven long-term biocompatibility and a modulus of elasticity between cortical and cancellous bone.
- Surface Ridges: resist implant migration while providing a broad surface area to prevent subsidence.
- Central I-beam Design prevents de-formation during placement, and provides spacious compartments for bone graft.
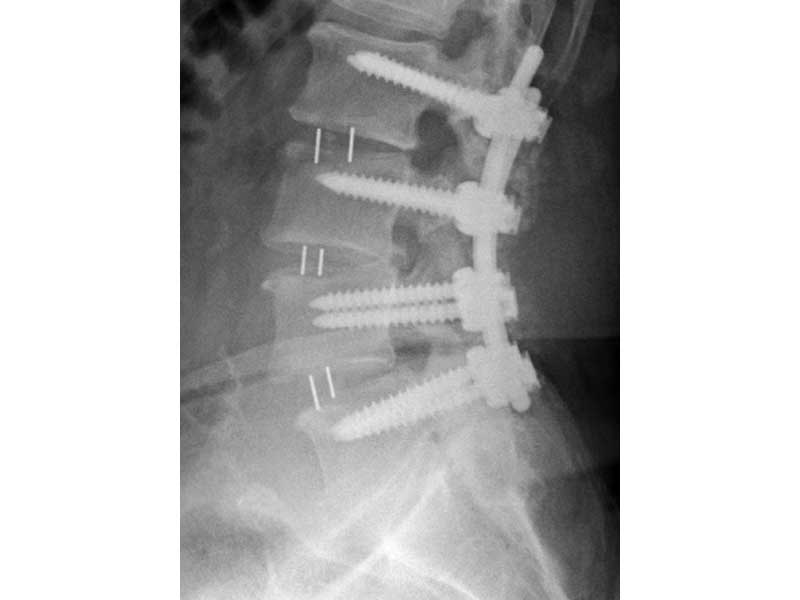
- Radiographic Markers: Embedded tantalum pins precisely reveal the implant position on x-ray without obscuring the view of the healing fusion.
- Surgeon Flexibility: straight and crescent options are included in a single case with intuitive and ergonomic insertion instruments designed for safe and efficient implantation.
- Range of Sizes: available in 7mm - 15mm anterior heights in standard 1mm increments.
- 8 Degrees of Lordosis provides for restoration of normal sagittal alignment when placed anteriorly, and for coronal correction when placed asymmetrically.
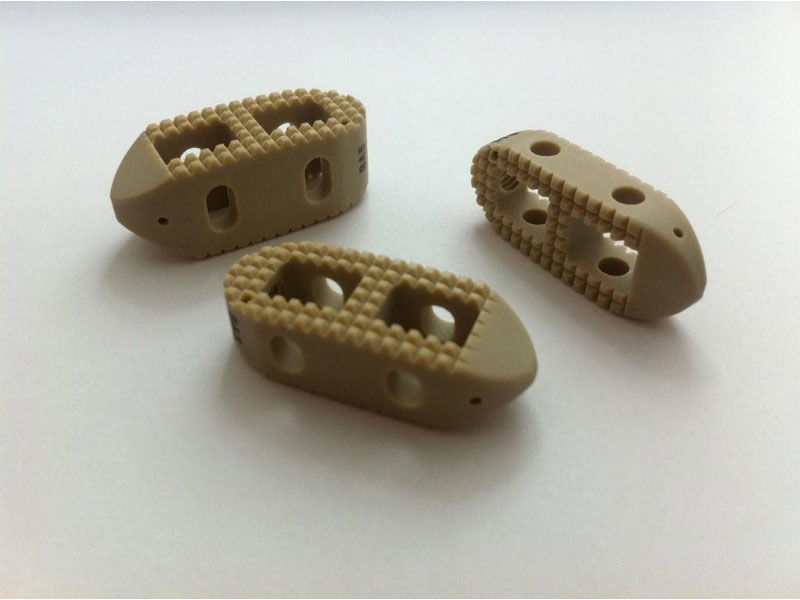
- Offered in two footprints for optimal flexibility:
- Crescent Lumbar Spacer:
- Curved Contour facilitates placement at the periphery of the disk space for contact with the most dense endplate bone.
- 10 and 25 Degree Insertion Angles allow flexibility in surgical approach to minimize neural retraction.
- Tapered Ends facilitate insertion and prevent endplate violation.
- Straight Lumbar Spacer:
- Rectangular Shape Suited for oblique placement for single implant and parallel bilateral placement for two implants.
- Dimensions 11mm x 30mm sizing allows for MIS insertion through tubes, minimal neural retraction, and superior endplate surface area contact.
- Crescent Lumbar Spacer:
Click on a thumbnail above to zoom image
-
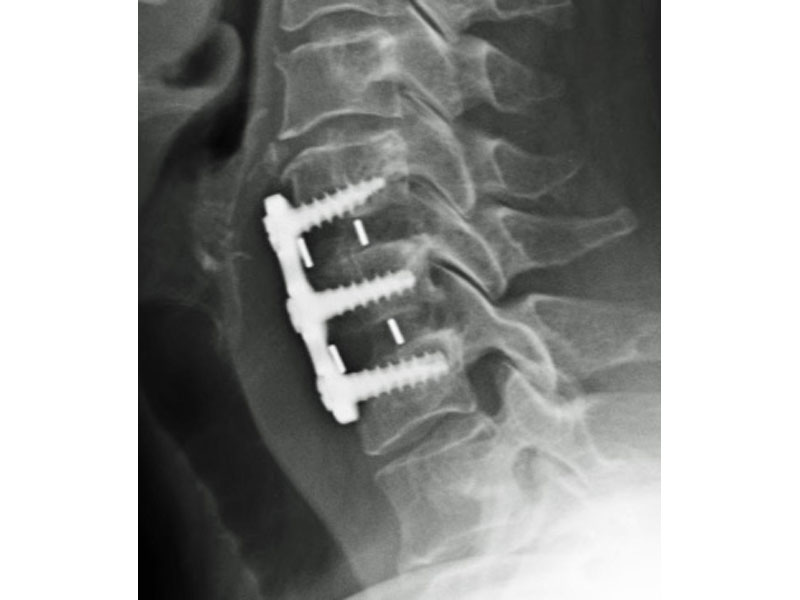
Calvary's Cervical Inter-body Fusion Device
- Anatomic Contour provides broad endplate coverage to minimize the potential for subsidence.
- Surface Ridges: resist implant migration while providing a broad surface area to prevent subsidence.
- 5 Deg Lordosis provides for restoration of normal sagittal alignment.
- Range of Sizes: 5mm, 6mm, 7mm, 8mm, 9mm anterior heights.
- PEEK-OPTIMA Polymer Material: PEEK-OPTIMA is a safe, biocompatible and stable polymer used in thousands of FDA cleared and CE-marked implanted devices since its introduction in 1999. It combines optimal biomechanics with modulus between cortical and cancellous bone, and established biocompatibility.
- Large Graft Containment Capacity: allows for housing a large volume of cancellous bone graft or extenders to promote rapid fusion.
- Radiographic Markers: Embedded tantalum pins precisely reveal the implant position on x-ray without obscuring the view of the healing fusion.
PEEK-OPTIMA and Invibio are registered trademarks of Invibio Ltd in the United State, European Community, and elsewhere.